In order to combat terrorism and prevent the illicit manufacture of explosives, the European Union has developed a specific regulation that regulates the marketing, sale and use of substances considered to be explosives precursors Regulation (EU) 2019/1148. This article aims to resolve any doubts that may arise in relation to SUCITESA products
What is an explosives precursor?
An explosives precursor is any chemical substance that alone or in combination with others can be used for the illegal manufacture of explosives. The regulation distinguishes between two types of substances:
- Restricted precursors: substances listed in Annex I and mixtures in which this substance is present, which must not be made available to the general public unless their concentration is equal to or lower than the limit values listed.
- Regulated precursors: substances and mixtures of these substances listed in Annex I or II with a concentration above 1%.
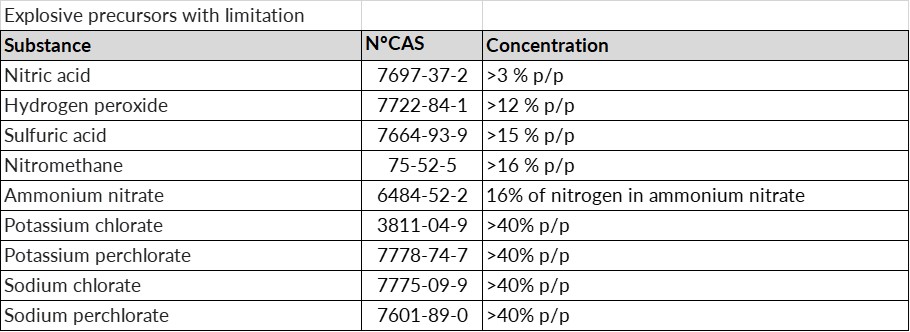
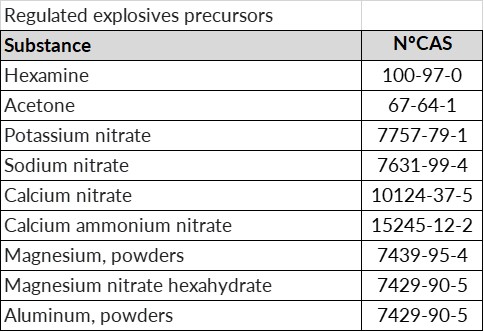
In any case, if a suspicious transaction, disappearance or theft of any type of substance considered an explosives precursor is detected, we must notify the competent authority within 24 hours.
What are SUCITESA’s obligations?
For the purposes of the regulation, SUCITESA is considered an economic operator and must inform the entire supply chain of the obligations generated, leaving a record of its compliance.
You can find this information, where applicable, in Section 15 of the safety data sheets.

Informations réglementaires figurant dans nos Fiches de Données de Sécurité concernant les précurseurs d’explosifs
Do professional users who require the use of any of this restricted substance need a licence?
Professional users who have a demonstrable need to use a restricted explosives precursor do not require a special licence as the use of this substance is considered essential for cleaning and disinfection.
However, the economic activity of the company, the fact that the person acquiring the precursor is a professional and the use to which the substance is to be put must be proven.
For further information, please click on the following link:






