When we hear the word cosmetic, the first thing that comes to mind are products for the care or beautification of the skin at home or the products used in beauty salons, but did you know that the term is much broader and more Does it also affect some products that we regularly use in professional hygiene?
The exact definition of a cosmetic product is “any substance or mixture that is intended to be brought into contact with the superficial parts of the human body (epidermis, hair and capillary system, nails, lips and external genital organs) or with the teeth and mucous membranes with the exclusive or main purpose of cleaning, perfuming, modifying their appearance, protecting them, keeping them in good condition or correcting body odors”.
This definition includes perfumes and fragrances, decorative cosmetics, skin care, hair care, and personal hygiene products such as moisturizing creams, deodorants, hand gels, shower gels, shampoos, conditioners, toothpastes, etc.. domestic, for sale in pharmacies and parapharmacies to shampoos, shower gels and hand gels for industrial or professional use.

All cosmetic products marketed in Europe must comply with specific regulations: Regulation 1223/2009, Commission Decision on Regulation 1, Regulation 655/2013 Common Criteria, successive Regulations that modify the Annexes and specific legislation of each country.
This regulation applies to any personal hygiene product, from the hand soap we use at home to the hand gel we use after going to the bathroom at a shopping center or restaurant.

The fundamental principle of the regulation is that cosmetic products placed on the market must be safe for human health.
What does this regulation imply?
Specific labelling: the label of this type of product must indicate the name or company name of the manufacturer, the nominal content, the date of minimum durability or PAO, precautions for use, lot, function of the product and the list of ingredients.
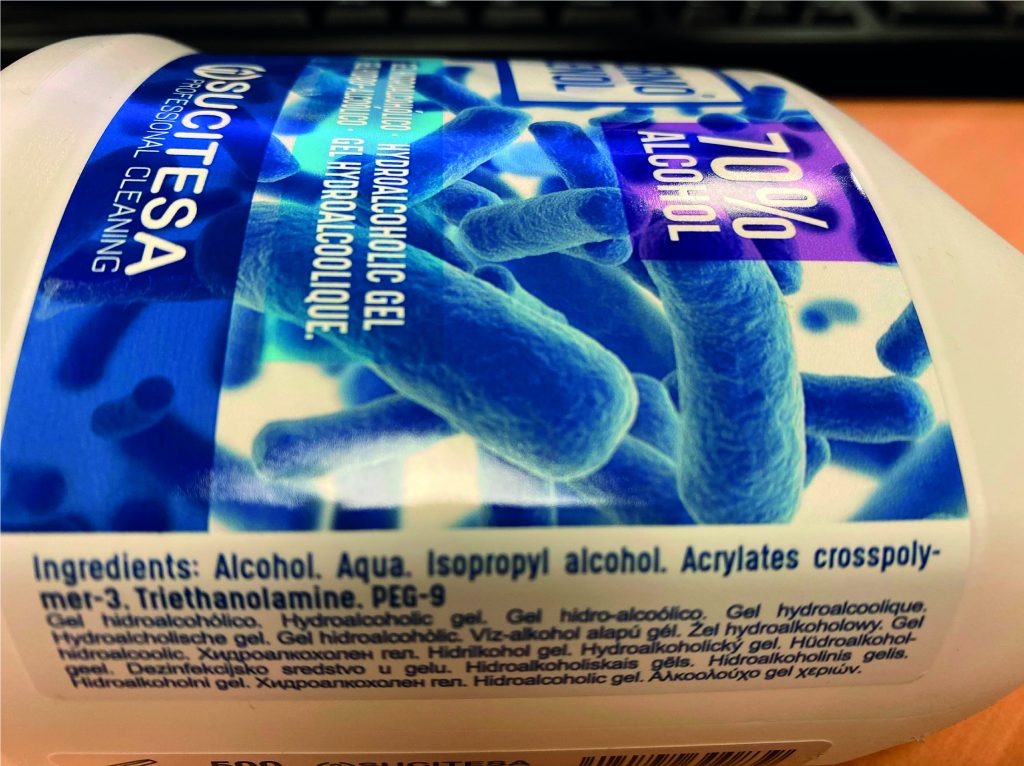
Ingredients: the ingredients cannot be anything, they must be of a cosmetic nature. The list of ingredients is indicated in the International Nomenclature of Cosmetic Ingredients and they are ordered according to their amount in the composition of the product, those that are in the highest proportion appear at the beginning, and those that are found in smaller amounts appear towards the end.
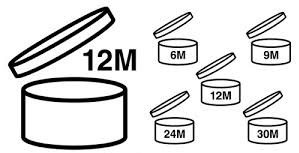
Useful life of the product: the period of time in which the cosmetic maintains its quality and integrity within its container must be indicated. Establishing this period of time implies stability tests at the laboratory level: exposure to changes in humidity, exposure to light, temperature, etc. to ensure that the product remains in good condition throughout its useful life.
Manufacturing: the regulations require cosmetics to be manufactured in accordance with GMP good manufacturing practices, requiring a quality guarantee during manufacturing:

Manufacture in a Clean Room approved by Health and periodically inspected to guarantee compliance with regulations.
A clean room is a room in which the quality of the air and parameters such as temperature, humidity or pressure are controlled. It is built with non-porous materials, waterproof, easy to clean, resistant to disinfectant agents.
Do you want to know our WHITE ROOM? Here we manufacture our range of cosmetic products
Microbiological controls of all products and manufacturing batches to ensure that they are free of microbiological contamination and are safe for users. Without issuing the batches until the results of the controls are available (quarantine).

Product Information File: it is the report that collects all the relevant information about a cosmetic product before it is marketed and shows that it has been subjected to an evaluation of its safety for human health.
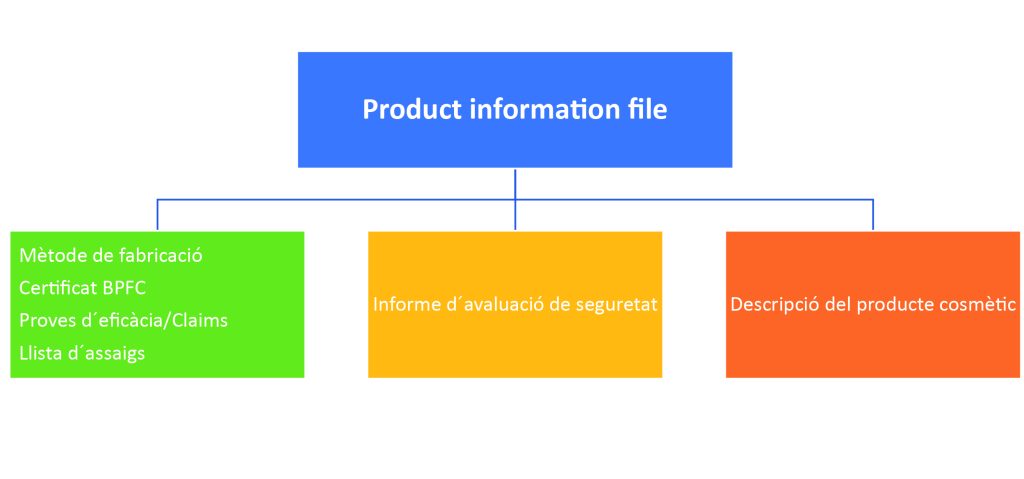
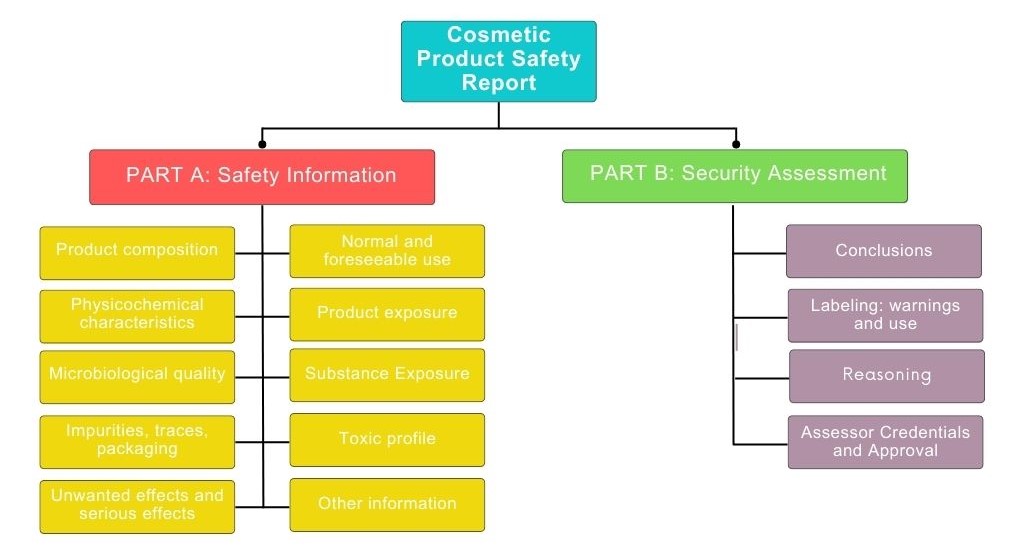
Finally, cosmetic products are subjected to an exhaustive control called cosmetovigilance where all unwanted effects after normal or foreseeable use of cosmetics are collected, evaluated and monitored. Cosmetovigilance is also responsible for disseminating information related to these effects.

Most companies manufacture and market cosmetics in the proper way, but unfortunately there are others that do not.
Don’t take unnecessary risks! Consult our range of products for Personal Hygiene






